Cancer researcher Rita Fior uses zebrafish to study human cancer. Though this may seem like an unlikely match, her work shows great promise with forthcoming applications in personalised medicine.
The basic principle of Fior's approach relies on transplanting human cancer cells into dozens of zebrafish larvae. The fish then serve as "living test tubes" where various treatments, such as different chemotherapy drugs, can be tested to reveal which works best. The assay is rapid, producing an answer within four short days.
Some years ago, when Fior was developing this assay, she noticed something curious. "The majority of human tumour cells successfully engrafted in the fish, but some tumours didn't. They would just disappear within a day or two. However, when I treated the transplanted fish with chemotherapy, these tumours would not disappear anymore. They engrafted much more”, she recalls.
This seemingly paradoxical observation triggered a new working hypothesis. "Chemotherapy suppresses the immune system", Fior explains. "If the tumour is rejected under normal conditions, but thrives in immuno-suppressed animals, then this points towards a new explanation: the fish's immune system is actively destroying the cancer cells. Whereas in the ones that implant well, the tumour is able to suppress the fish’s immune system.”
Soon after, Fior, together with Vanda Povoa, a doctoral student in her lab at the Champalimaud Centre for the Unknown in Portugal, set off on a new research project. It's main conclusions, published today (February 19th) in the journal Nature Communications, advance our understanding of how cancer-immune interactions may lead to immunotherapy resistance and tumour growth. In the long run, these results may contribute to the development of new treatments and diagnostics.
Good Cop
Following this serendipitous observation, the researchers began systematically investigating why certain tumours are eliminated while others survive.
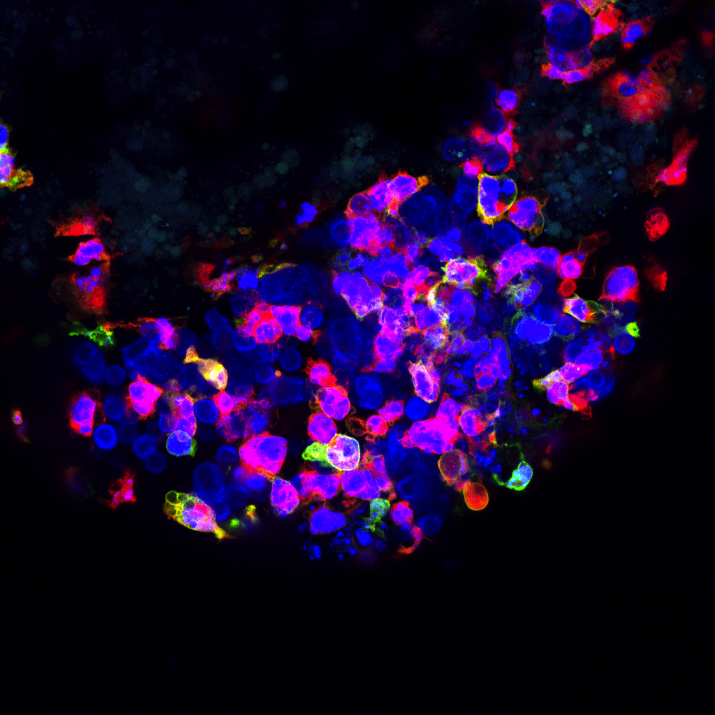
They focused on a pair of human colorectal cancer cells that were derived from the same patient but showed these contrasting behaviours. One was derived from the primary tumour and was constantly rejected from the fish; whereas the other was derived from a lymph node metastasis but implanted very efficiently.
They started by characterising the immune cells that were summoned to the tumour site. Specifically, they zoomed-in on cells of a subsystem called innate immunity.
"Contrary to mature zebrafish, the larvae only have innate immunity, which is the body's first line of defence. This offers a vantage point to study the role of innate immune cells in cancer, which is not very well understood", Fior explains.
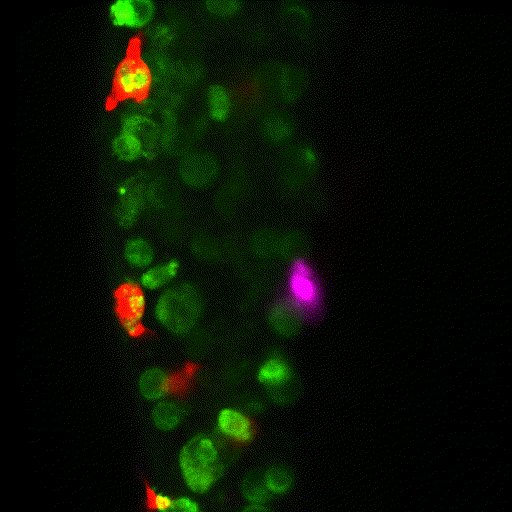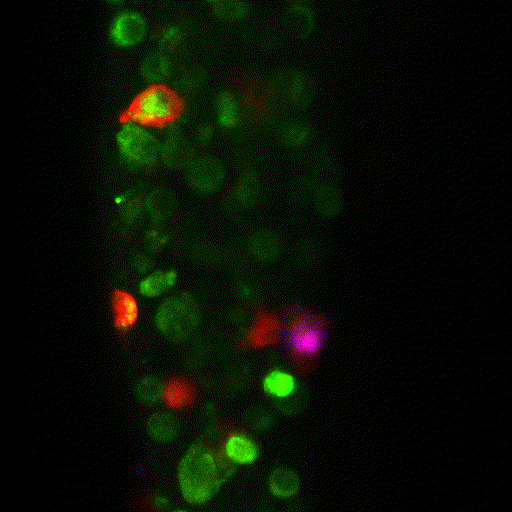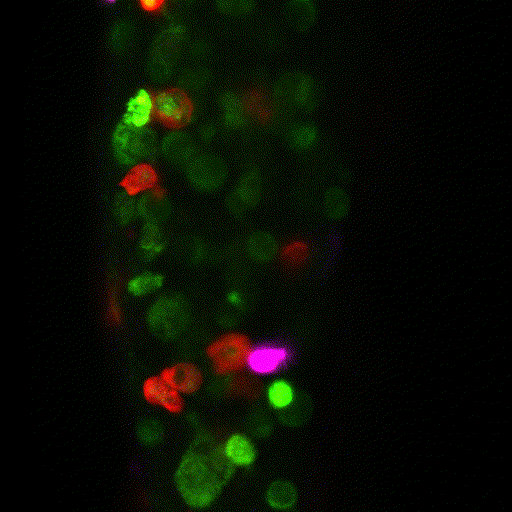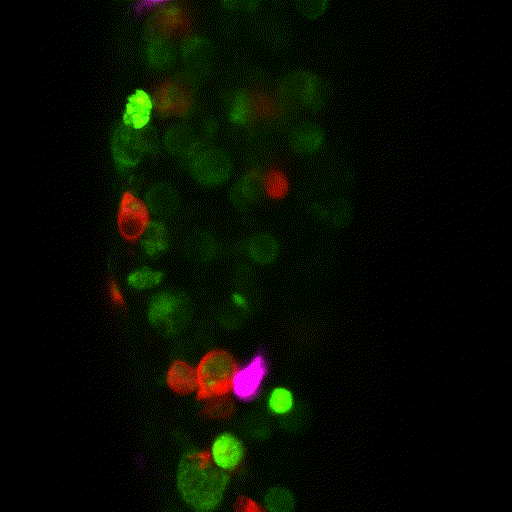
The team proceeded to quantify the number and type of innate immune cells in the tumour microenvironment. The primary tumour [which gets rejected] was swarming with innate immune cells. But in contrast, the metastatic tumour that implants well, showed very sparse numbers of innate immune cells.
This result indicated that the researchers' hunch was correct. But to be sure, they had to artificially reduce the number of innate immune cells in the fish using selective genetic and chemical approaches. As expected, this manipulation "saved" the cells from being rejected.
Together, these results show a clear role for the innate immune system in eliminating tumour cells. But then, if the immune system is so good at getting rid of "fresh" cancer cells from the primary tumour, why would metastasis happen?
Bad Cop
"The reason is that the relationship between cancer and the immune system is far from static", says Fior. "At the beginning, cancer cells may simply try to hide from the immune system. But with time, they learn how to confuse and finally corrupt immune cells. This evolution happens through a dynamic process called 'Immunoediting'. If the process is successful, the corrupted cells begin supporting the tumour in many ways, including sending away other immune cells that could vanquish the tumour."
Is the innate immune system per se able to do cancer immunoediting? "Our results show that yes, it does indeed. This is the second study, to our knowledge, that shows this phenomenon", adds Povoa.
The researchers observed that tumour cells not only recruit different numbers of innate immune cells but can also change their function. Instead of fighting the tumour, macrophages began supporting and protecting it. Alarmingly, the researchers demonstrated that this transformation happens very quickly.
"Even though most cells from the primary tumour are rejected within a day or two, some survive. When we transplanted this small group of survivors back into the fish, we discovered that they had already acquired immune-editing capabilities! In fact, they engrafted almost as well as cells from metastatic tumours", Povoa points out.
The researchers also compared the genetic profile of metastatic and primary tumour cells and identified several interesting features. "We now have a list of candidate genes and molecules that we plan to study. We hope that by pinpointing the mechanism by which cancer cells suppress and corrupt the innate immune system we will be able to find ways of blocking this process", Fior adds.
Giving immunotherapy a boost
Fueled by this exciting set of results, Fior and Povoa are full of plans for the future. "There are so many things we can do", says Fior. "For instance, we now know that our zebrafish assay can tell if the tumour environment is immunosuppressive in just a few days. Immunotherapy is less likely to be effective in these cases. Therefore, our assay may become useful to help determine which patients will benefit mostly from immunotherapy ."
Another angle the team is thinking about is the development of new immunotherapy approaches. "The majority of immunotherapy drugs do not rely on innate immunity. They boost other immune subsystems. But as we saw, innate immunity has a great capacity for fighting cancer. Therefore, identifying the mechanisms that amplify this effect will allow us to devise new potential therapies, which could be combined with existing ones to increase their efficacy.", she concludes.
By the CCU Communication, Outreach and Events Team.
Original Paper published on Nature Communications. DOI: 10.1038/s41467-021-21421-y