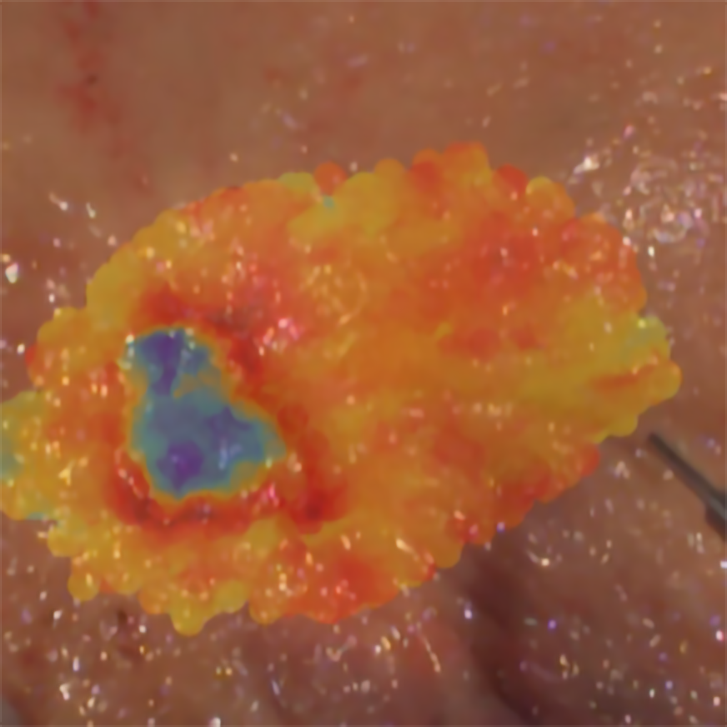
A portion of human gut – just removed from the abdomen of a colon cancer patient and opened in half so as to give access to its inner mucosa, – sits on the countertop. A technician starts to scan it with the tip of an optical fibre, hovering over the surface of the sample without touching it. On the scanned zones, streaks of various colours, as if the tissue is being “painted” by the laser beam coming out of the fibre optic.
It so happens that the light from the laser naturally makes the sample tissue emit fluorescence. And that the fluorescence contains crucial information about the tumour that is being scanned. Thus, in just a few minutes, the technician can paint (in false colours, corresponding to a scale of numeric values) not only the tumour, but also where its margins end and healthy tissue begins.
In a not too distant future, believes 36 year-old biomedical engineer (with a PhD in photonics) João Lagarto, who coordinates the Biophotonics Platform at the Champalimaud Foundation, this same procedure could be performed, in vivo, on patients undergoing surgery – before removing the tumour –, to ensure that surgeons feel confident that they are removing the whole tumour, without leaving anything behind, thus substantially improving patient prognosis.
João Lagarto arrived at the Champalimaud Foundation in September 2020, where he started by participating in the Watch & Wait programme (W&W) [link to article here], which is currently being applied, in the Digestive Unit, to certain rectal cancer patients. “Our initial work was focused on validating our technique for future implementation in the W&W programme – that is why we now work with colon and rectum tissues”, he explains. But the interest of his fluorescence-based methodology rapidly expanded to other applications with high diagnostic and therapeutic potential.
For now, João Lagarto and his colleague Ignacio Herrando, a colorectal surgeon who liaises between the clinic and the Biophotonics Platform, are taking the first steps towards validating this “optical biopsy”, analysing and “painting” tissue that has already been removed from the patient. And the first results are very promising.
Exploring the natural fluorescence of biological tissues
Body tissues contain certain molecules, called fluorophores, that emit fluorescence when stimulated by light. “Certain fluorophores have very specific functions in the tumour’s biochemistry, which can be studied with light”, says João Lagarto. Using laser light of various colours, and an optical fibre, these researchers are able to capture, through light-sensitive detectors (and the same optical fibre that sends the laser light to the tissue) the fluorescence emitted by the fluorophores stimulated by the laser. And from there, they can extract certain physical properties of the emission, such as its (true) colour, its intensity and most of all its “lifetime”, that is, the time it takes for the fluorescence to decline and disappear.
But what does all this have to do with cancer? “Previous studies have shown that there is a correlation between fluorescence lifetimes and the cancerous process”, replies João Lagarto.
The technique these researchers use to measure fluorescence lifetimes is already used. It is called TCSPC (time-correlated single-photon counting). “The originality here is that we are the only ones who use this technique in a clinical setting and in a high-luminosity environment”, João Lagarto points out.
Why? Because the intense luminosity that reigns in an operating room represents a big hurdle when you want to measure fluorescence in real time in this setting, given that the fluorescence emitted by tissue fluorophores is typically very weak, João Lagarto explains. And here he quickly adds that he cannot provide further technical details, in particular about how they solved the problem, because there are various pending patents concerning this part of the work.
Additionally, the laser light the team uses is low intensity, avoiding damage to the tissues under examination and measurement errors in fluorescence lifetimes – complications that could happen with more powerful lasers.
“Our aim is to be able to evaluate tissues during diagnostic endoscopies with our method, by superposing fluorescence images and endoscopic images”, the researcher continues. And following oncological treatment with chemotherapy or radiotherapy (or a combination of both), we also want to be able to evaluate, again by endoscopy, the recovery of the tissue.”
What about during surgery? “At present, we only work with samples that have just been removed from a patient and have very visible tumours. We do this before they are sent on for histopathological analysis”, says João Lagarto. As already mentioned, in two or three minutes, the team would be able to give feedback to the surgeon about the localization and the margins of the tumours.
Once validated and integrated into the surgical protocol for this use, it could mean a big gain in terms of waiting times during surgery compared to histopathology. Today, histopathological analysis is used to identify the tumour and aid the surgeon’s decision-making, but “it is a time-consuming procedure and it can return inconclusive results. Therefore, it doesn’t always meet surgery needs”, says João Lagarto. Nevertheless, before the new methodology is approved for use in the operating room, there is a long way to go.
But it is a fact that this experimental procedure is already giving good preliminary results. “Until now, we have compared our fluorescence images of tissues extracted by surgeons with histopathology images of the same sample in 29 patients”, says João Lagarto, and in 24 of those cases we were able to detect the tumour. And given that in the remaining cases there was no tumour [something that can happen], this means that we detected the tumour in 100% of the cases. Indeed, one of the most interesting aspects is precisely that, when there is no tumour, the fluorescence signal tells us just that.”
However, at this stage, the researchers are not yet able to identify specific patterns within the extremely complex set of fluorescence signals they gather from each sample. The raw signals basically appear as a jumble of lights of different colours simultaneously emitted by a multiplicity of fluorophores, with very variable lifetimes. And everything happens, at the most, in a few nanoseconds from the arrival of the laser light to the tissue to the decline of the resulting fluorescence.
But still, João Lagarto considers that, in the future, with the help of artificial intelligence and machine learning, it will be possible to to analyse more meticulously the characteristics of the data, which on top of everything else also vary from organ to organ and depending on the colour of the laser that was used. “This will be one of the next steps in the validation process of our methodology”, he notes.
In any case, what matters most right now is that the value of the fluorescence lifetimes (measured in nanoseconds) that characterise an abnormal tissue are different from those that characterise a healthy tissue. “In the presence of inflammation, fibrosis, or a tumour, be it malignant or not, our measurements are able to detect those anomalies”, stresses João Lagarto. “More to the point, some of those values are always different from what they would be in a healthy tissue.”
The team is also engaged in a protocol with the Gynaecology Unit for the early detection of endometrial cancer during hysteroscopy and in a protocol aiming to characterise pancreatic cysts and pre-cancerous lesions of the pancreas. This second protocol will be carried out at the new Botton-Champalimaud Pancreatic Cancer Centre. “We work in collaboration with the clinical units and the pathology service. Our goal is to serve the clinicians and the patients”, says João Lagarto.
When does the researcher think his method will be ready for use in vivo in the operating room? “I hope that until the end of 2023 we will have the first case-study, in vivo, in open surgery”, he replies. “Concerning laparoscopic and robotic surgery, more time will be needed: at least two or three years. That is because in those cases, we will have to integrate our approach with already existing clinical equipment.”
Image caption
Fluorescence image obtained by the team, with the tumour (colon) appearing in blue hues. At the bottom right corner, the tip of the fibre optic “scanning” device is visible.
Image Credit
João Lagarto and Ignacio Herrando
Text by Ana Gerschenfeld, Health & Science Writer of the Champalimaud Foundation.